Conductivity Meter Calibration Procedure
What is Conductivity?
The conductivity of a solution is determined by the ability of its ions to conduct an electric current. Pure water is not a good conductor but a poor conductor of electricity because its ability to ionize is very limited. The more ions are present in the water, the higher its conductivity.
The conductivity electrode cell is applied with an AC voltage immersed in a sample solution whose conductivity is to be determined. The amount of voltage drop is caused by the resistance of the sample solution, voltage drop is used to find out the sample conductivity.
The conductivity cell with constants 1.0, 0.1, and 0.001 depends on the conductivity range of the sample solution.
The basic unit of conductance is mho or Siemens (S). Since Siemens is a large unit, samples are measured in µS /cm (micro Siemens) is used to describe the conductivity of a solution.
Basics of Conductivity Meter
Conductivity is an important quality parameter, its equipment calibration is a part of functional verification. The calibration frequency is different for each situation. It depends on how many times the meter is used and the amount of dirt on the conductivity sensor.
The higher that number, the more times the meter is required to calibrate. It is advised to calibrate the conductivity meter a minimum of twice a month.
Following are the situations, when calibration is required
- If the electrode is a new one.
- An electrode that has not been used for a long time (inactive electrode).
- After electrode cleaning.
- When using the electrode in a highly concentrated solution.
Tools Required for Calibration
- A small 1 or 1.5mm flat knob-type screwdriver to adjust the conductivity meter.
- Distilled water.
- A pair of disposable clear plastic cups.
Calibration Procedure of a Conductivity Meter

Before beginning the calibration of the conductivity meter, let us take calibration solutions 1413 uS/cm in a disposable cup or a beaker.
Care should be about 3 cm of liquid in the beaker to cover the electrode (the glass sphere at the tip of the electrode).

Put the meter in an OFF state and submerge the electrode in the calibrator solution that is poured into the beaker.
Turn the meter ON and wait for some time for the reading to stabilize or stop changing.
If the display reads 1413 uS/cm, the meter does not need to be adjusted, otherwise use the screwdriver to adjust the small screw on the back of your electro-conductivity meter until the reading conductivity of the calibration solution.
Now that if have adjusted it, turn OFF the conductivity meter, rinse the electrode with distilled water or tap water and dry it with a clean towel or cloth.
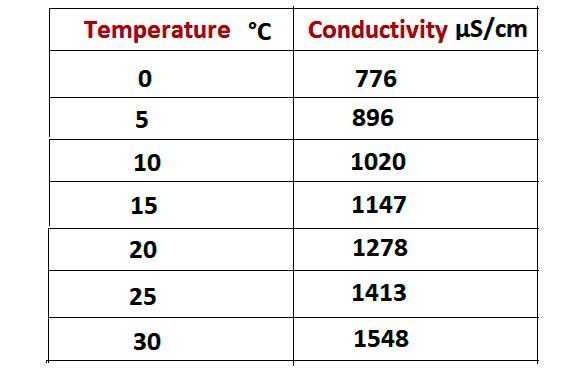
Following are the uS/cm logs based on different temperatures.
Keywords
Distilled water
Water is subjected to an evaporation and re-condensation process. This process produces very pure water since all dissolved solids are retained in the first step.
Solution
In chemistry, a solution is a homogeneous mixture of solvent and one or more solutes.
Solvent
A solvent (a higher amount) is a substance in which a solute dissolves and forms a solution. Generally, the solvent is the component that is found in a large proportion of the solution. Water (universal solvent) and oil are examples of a solvent.
Solute
A Solute (lower amount) is a substance that dissolves in a solvent thereby forming solutions. General examples of solutes are sugar and salt, they dissolve in water (solvent) to form solutes.













Comments
4
very good
Awesome man, I really appreciate that
If the temperature is above 30 what will be the range can you pls help me on this.
32-
34-
36-
38-
40-
42-
If I use a conductivity meter, Is it possible to measure a particular metal ion concentration in the mixture while an adsorbent is added in the aqueous solution?