pH Meter Calibration Procedure
What is pH Measurement?
pH is the universal unit for measuring the levels of acidity or alkalinity of a solution. The pH values that comprise this measurement are part of a simplified scale with values between 1 and 14.
In which 1 is a “very acidic character” and 14 is a “very alkaline character”. Whenever there is a balance in the values, the value is pH7 and when this occurs we say that the sample has neutral pH.
pH measurement of a solution determines the nature of a solution which may be an acidic or alkaline state.
Basics of pH measurement
The pH measurement is found widely in power plants, chemical industries, and laboratories. The determination of pH consists of a combination electrode or a measurement plus a reference electrode.
The reference electrode must present a constant value of the potential regardless of the pH value, the solution to be studied, and the measurement conditions.
The measurement electrode, for its part, must respond only to the activity of the H+ ions in the solution.
The system is complemented by the electronics associated with the measurement and reference electrodes, which allow us to read the reading in units of pH or millivolts, as well as automatic or manual temperature compensation, zero adjustments, and slope.
The pH measurement scale extends from 1 to 14. The lower part of the scale corresponds to strongly acidic solutions, while the upper values of the range are related to strongly basic solutions.
Tools Required for pH Calibration
- Demineral water bottle with cup
- Three buffer solutions of pH4, pH7, and pH9
Pre-requisites and Precautionary Measures
A buffer solution is to be prepared with a buffer substance that must be within the period before its expiration date.
A buffer solution that has been left in the air for more than one hour should not be used.
Use good practice in the use of buffer substances to avoid contamination as much as possible once the bottle is opened. Do not carry out measurements directly from the bottle, and never return to the bottle the remaining fraction of solution used in each fine-tuning process of electrodes and pH meter.
Keep buffer substances in a cool and dimly lit place.
Thoroughly clean the contacts of the electrode terminals to eliminate false contacts.
Observe all the care indicated by the manufacturers for the electrodes, and immerse them in the buffer solution in such a way that the entire membrane is submerged in the solution.
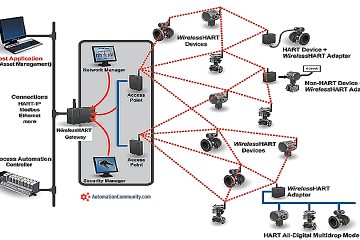
Calibration Setup

pH Meter Calibration Procedure
The pH meter calibration process is to be carried out in a usual place of work and within the appropriate temperature ranges.
The following is the calibration procedure
1) The pH measurement system must be running for at least 30 minutes before starting the calibration process.
2) Adjustment of the manual temperature compensation to the value at which the adjustment of the buffer solutions will be carried out. This value is determined by measuring the temperature of the buffer solutions with the thermometer.
3) Examine the electrode to verify that there is no defect or the presence of air bubbles inside, if there are any, shake the electrode in a similar way to clinical thermometers to lower the temperature.
4) Clean the electrode externally with plenty of distilled water.
5) Immerse the electrode (or electrodes if they are not combined) in the buffer solution at a controlled temperature. The solution used should be the one with the closest pH to the internal pH of the glass electrode, which is usually pH 7.
6) Wait for thermal equilibrium for approximately 1 minute. Once the reading has stabilized, activate the calibration-standardization-asymmetry neutral point control until an indication of the pH of the buffer solution is obtained.
7) Remove the electrode from the solution and wash it with abundant distilled water or with the buffer solution that will be used next. The electrode can be dried without rubbing.
8) Immerse the electrode in another glass containing another buffer solution with a pH different from the previous one (pH 4 is usually used).
9) Wait for thermal equilibrium for approximately 1 minute.
If adjustment is required using another calibration point (for example, pH 9), points 7) to 9) must be repeated.

Keywords
Electrolyte
The electrolyte is a solvent that dissolves in water that can be subjected to electrolysis. In electrolytes, free ions act as electrical conductors. The most common type of electrolyte is potassium chloride (KCL).
Reference electrode
A reference electrode is stable and known to have an equilibrium potential. It is an electrode used to reliably and reproducibly measure the potential applied to the working electrode.
Combination electrode
The measurement electrode and the reference electrode are combined in one unit with the combination electrode. It is the most common electrode used to measure pH.
Electrode body
It may be plastic or glass. Glass electrode consists of membrane material, not the external electrode body.